Tosymra® delivers migraine pain relief in as little as 10 minutes with just one spray for some patients (13% vs. 5% for placebo).*1-3
Efficacy of Tosymra® is based on relative bioavailability to subcutaneous sumatriptan at a dose of 4 mg. In a clinical study, this dose of sumatriptan resulted in 57% of patients achieving pain relief at 2 hours vs. 21% for placebo.1
*Time to onset and degree of pain relief varies by patient.
Give your patients options
Oral therapy may not be the optimal treatment for every migraine. See why Tosymra® uses the novel ingredient Intravail®.
Tosymra® achieved peak plasma concentration
8x faster than Imitrex® nasal spray4
Mean sumatriptan plasma concentration-time profile
Results from a randomized, crossover, pilot pharmacokinetic study conducted in 18 healthy adults.4 In this study, three AEs occurred with Tosymra® (vomiting, paresthesia and burning sensation in nose). Three AEs occurred with Imitrex® (sumatriptan) 20 mg nasal spray (nausea and throat irritation [two events]). All events were mild or moderate in intensity.5

Tosymra® uses the science of Intravail®
Tosymra® is the first and only triptan nasal spray to use the novel ingredient Intravail® to enhance drug absorption across the nasal mucosa.6
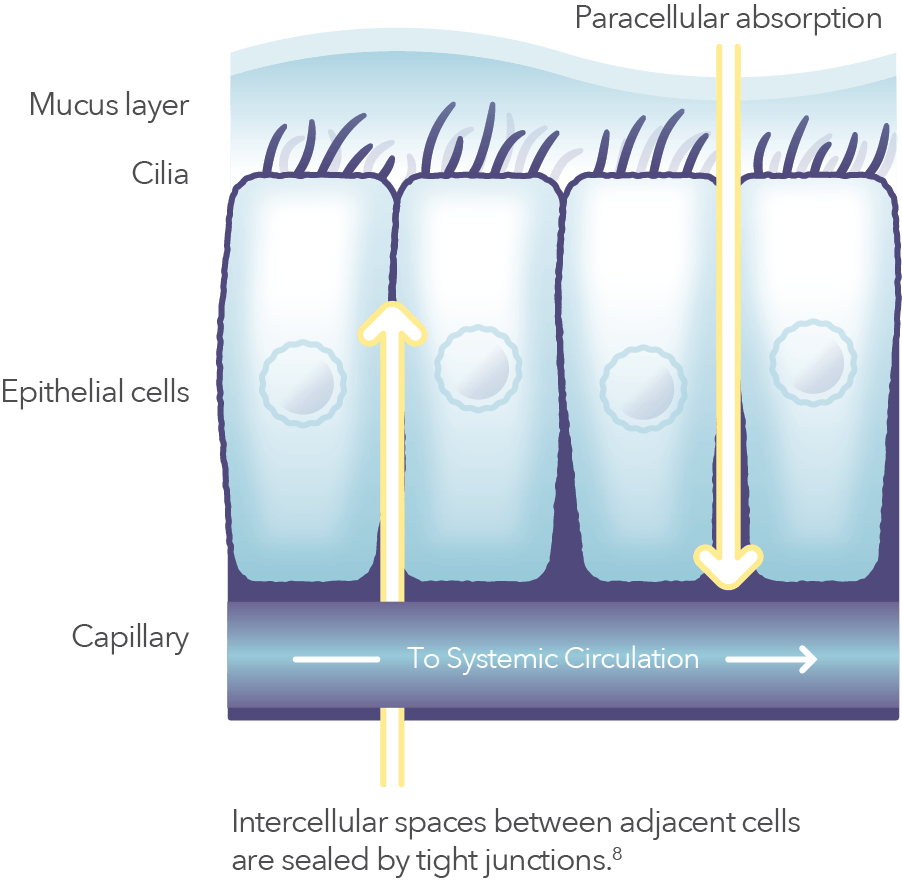
Controlled transient permeation of the nasal mucosa6
Intravail® is thought to facilitate paracellular absorption by transiently relaxing tight junctions, allowing drug access to systemic circulation, among other mechanisms.6-8
Nasal mucosa

Tosymra® is a sumatriptan nasal spray with mist-like administration
The safety profile of Tosymra® is generally consistent with that of subcutaneous injectable sumatriptan.
ADVERSE REACTIONS:
Most common adverse reactions (≥5% and > placebo) with sumatriptan injection were tingling, dizziness/vertigo, warm/hot sensation, burning sensation, feeling of heaviness, pressure sensation, flushing, feeling of tightness, and numbness.
Additional common adverse reactions with Tosymra® include the local irritative symptoms of application site reaction, dysgeusia, and throat irritation.
Tolerability
In a 6-month open-label, repeat-dose safety study designed to assess the safety and tolerability of Tosymra® in 167 adult patients, Tosymra® was well-tolerated, with a low rate of triptan-related adverse events (TEAEs).9 Over the course of the 6-month study, 3,292 doses of Tosymra® were used to treat 2,211 migraine attacks.10
| Most common TEAEs (≥5% of subjects) |
Patients† n(%)9 |
Total events n5 |
Incidence per individual dose5,9 |
|---|---|---|---|
| Application site pain* | 51 (30.5%) | 568 | 17.3% |
| Dysgeusia | 35 (21.0%) | 232 | 7.0% |
| Upper respiratory tract infection | 18 (10.8%) | 24 | 0.7% |
| Nasopharyngitis | 12 (7.2%) | 13 | 0.4% |
| Sinusitis†† | 11 (6.6%) | 12 | 0.4% |
| Application site reaction | 9 (5.4%) | 32 | 1.0% |
†Percent of patients experiencing the event at least once during the course of the study.
††Only one event of sinusitis was considered “possibly related” to Tosymra®.
- 5 patients (3%) discontinued due to adverse events.10
- Overall, 2.9% of doses were associated with a triptan-related TEAE.10
- The majority of triptan-related adverse events were mild; none were severe.9

Making Migraine Our Priority
Tonix Medicines, Inc. takes pride in providing attentive customer service, having strong industry relationships and being dedicated to uninterrupted supply.
Tosymra® continues our commitment to ensure you have a variety of medication options that can address the unique needs of migraine patients.
Want more information?
Sign up today to learn more about Tosymra®.
References:
- Tosymra [package insert]. Maple Grove, MN: Upsher-Smith Laboratories, LLC: 2021.
- Mathew NT, et al. Dose ranging efficacy and safety of subcutaneous sumatriptan in the acute treatment of migraine. US Sumatriptan Research Group. Arch Neurol. 1992;49(12):1271-1276.
- Wendt J, et al. A randomized, double-blind, placebo-controlled trial of the efficacy and tolerability of a 4-mg dose of subcutaneous sumatriptan for the treatment of acute migraine attacks in adults. Clinical Therapeutics. 2006;28(4):517-526.
- Munjal S, et al. A randomized trial comparing the pharmacokinetics, safety, and tolerability of DFN-02, an intranasal sumatriptan spray containing a permeation enhancer, with intranasal and subcutaneous sumatriptan in healthy adults. Headache. 2016;56(9):1455-1465.
- Data on file. Tonix Medicines, Inc., Chatham, NJ.
- Maggio ET. Intravail®: Highly effective intranasal delivery of peptide and protein drugs. Expert Opinion Drug Delivery. 2006;3(4):529-539.
- Maggio ET, Pillion DJ. High efficiency intranasal drug delivery using Intravail® alkylsaccharide absorption enhancers. Drug Deliv Transl Res. 2013;3(1):16-25.
- Ghadiri M, Young PM, Traini D. Strategies to enhance drug absorption via nasal and pulmonary routes. Pharmaceutics. 2019;11(3):113.
- Munjal S, et al. A multicenter, open-label, long-term safety and tolerability study of DFN-02, an intranasal spray of sumatriptan 10 mg plus permeation enhancer DDM, for the acute treatment of episodic migraine. J Headache Pain. 2017;18(1):31.
- Halvorsen MB, et al. Triptan-related adverse events in a multicenter, open-label, long-term, safety study of DFN-02 (Tosymra®; an intranasal spray of sumatriptan 10 mg plus permeation enhancer DDM), for the acute treatment of migraine. PAINWeek Conference 2019; September 3-7, 2019; Las Vegas, NV.